|
| Home | Crystal | jmol | jPOWD | Chem | X Ray | Dana | Strunz | Properties | A to Z | Images | Share | News | Help | About |
Unit Cell Dimensions
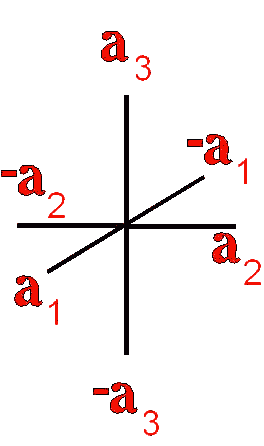
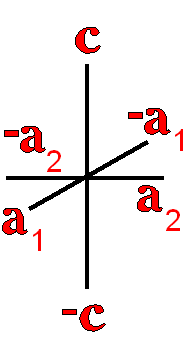
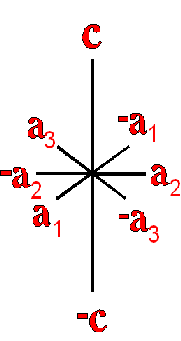
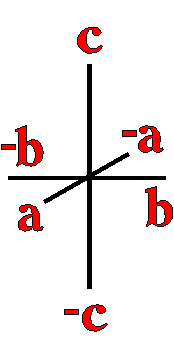
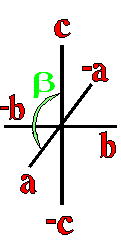
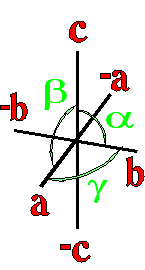
Definition"The unit cell of a mineral is the smallest divisible unit of a mineral that possesses the symmetry and properties of the mineral. It is a small group of atoms, from four to as many as 1000, that have a fixed geometry relative to one another. The atoms are arranged in a "box" with parallel sides called the unit cell which is repeated by simple translations to make up the crystal. The atoms may be at the corners, on the edges, on the faces, or wholly enclosed in the box, and each cell in the crystal is identical. This is what was meant by an "ordered internal arrangement" in our definition of a mineral. It is the reason why crystals have such nice faces, cleavages, and regular properties." (Joe Smyth, University of Colorado) The dimensions of the unit cell give its volume and are calculated as follows:
Where a, b, and c are the unit cell axes dimensions and α, β, and γ are the inclination angles of the axes in the unit cell.
Den(Calc) =[Molecular Weight]*[Z])/([Volume]*0.60225) Where: For Further Information on Unit Cell DimensionsSearch the Mineralogy DatabaseSearch the WebUniversity of Colorado Caution: Slow loading page. |