|
| Home | Crystal | jmol | jPOWD | Chem | X Ray | Dana | Strunz | Properties | A to Z | Images | Share | News | Help | About |
General Cyanochroite Information
Cyanochroite Image

|
Comments: Pale blue crystal cluster of cyanochroite. |
Cyanochroite Crystallography
Mouse
drag1 - LMB Manipulate Structure
drag2 - RMB Resize/Rotate
Keyboard
S - Stereo Pair on/off
H - Help Screen
I - Data Info
A - Atoms On/Off
P - Polyhedra On/Off
B - Bonds On/Off
Help on Above
|
Physical Properties of Cyanochroite
Optical Properties of Cyanochroite
CI calc= -0.002 (Superior) - where the CI = (1-KPDcalc/KC)
KPDcalc= 0.22,KPDmeas= 0.2206,KC= 0.2197
Ncalc = 1.49
Calculated Properties of Cyanochroite
note: Specific Gravity of Cyanochroite =2.23 gm/cc.
Boson Index = 0.9986430857
U=PECyanochroite x relectron= 19.55 barns/cc.
GRapi = 257.62 (Gamma Ray American Petroleum Institute Units)
Concentration of Cyanochroite per GRapi unit = 0.39 (%)
Estimated Radioactivity from Cyanochroite 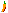 - barely detectable
- barely detectable
Cyanochroite Classification
Other Cyanochroite Information
1 - Am. Min. Crystal Structure Database
2 - Athena
3 - EUROmin Project
4 - Ecole des Mines de Paris
5 - GeoScienceWorld
6 - Google Images
7 - Google Scholar
8 - Handbook of Mineralogy (MinSocAm)
9 - Handbook of Mineralogy (UofA)
10 - MinDAT
11 - Mineralienatlas (Deutsch)
12 - Online Mineral Museum
13 - QUT Mineral Atlas
14 - Ruff.Info
Search for Cyanochroite using:
Visit our Advertisers for Cyanochroite :
A Bijoux Google Search for CyanochroiteAdam's Minerals Google Search for Cyanochroite
Cape Minerals Google Search for Cyanochroite
Dakota Matrix Minerals Google Search for Cyanochroite
Excalibur Mineral Corp. Google Search for Cyanochroite
Exceptional Minerals Google Search for Cyanochroite
John Betts Fine Minerals Search for Cyanochroite
McDougall Minerals Google Search for Cyanochroite
Mineral News Website Link
Rock and Mineral Shows Google Search for Cyanochroite
Weinrich Minerals, Inc. Google Search for Cyanochroite
Ask about Cyanochroite here :
Ask-A-Mineralogist from the Mineralogical Society of America
Mindat.org's Discussion Groups
Original Rockhounds Discussion Group
Rockhounds Discussion Group on Yahoo Groups
Mineral Discussion Forum from Fabre Minerals - also available in
Espaņol
Print or Cut-and-Paste your Cyanochroite Specimen Label here :
|
Cyanochroite K2Cu(SO4)2•6(H2O)Dana No: 29.03.06.02 Strunz No: 07.CC.60 Locality:
Notes:
|
